Q: Do the isothermal, isochoric, and isobaric relationships apply to points on the p-V diagram that are not directly connected? In the lab of Thermo Cycle (see figure), states 1 and 3 sit on an isotherm curve. Can I use the isothermal relationship, (p1)(V1)=(p3)(V3), even though there is a step in between these two points that deviates from the isothermal curve? More generally, does the isothermal relationship between two states at a same temperature hold true regardless of the path taken to get from one to the other, or does the path need to directly trace the isotherm for the relationship to be legitimate? Same question for isochoric and isobaric processes and their respective relationships.
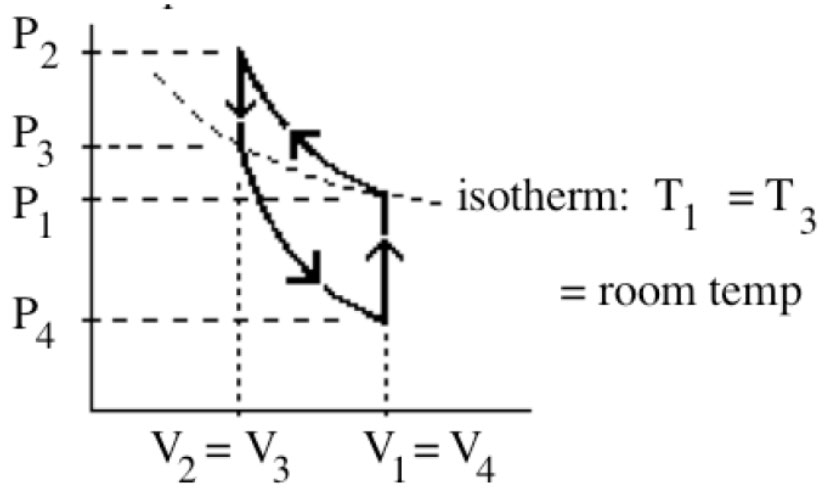
A: The simple answer is YES. Actually, we don’t have to consider any process connecting 2 states. As long as the 2 states are for a same gas system sealed in a container (mole number n is constant), we can simply apply Ideal Gas Law. For example, states 1 and 2 are two states on a same ideal gas cycle which may or may not be connected directly.
Ideal Gas Law: p1V1/T1 = p2V2/T2 = nR
If V1 = V2 (Isochoric), p1/T1 = p2/T2.
If p1 = p2 (Isobaric), V1/T1 = V2/T2.
If T1 = T2 (Isothermal), p1V1 = p2V2.
