Q: When looking at the formula Eth=W+Q, what is the difference between Work and Heat? Aren’t they both energy? If possible, please provide an example of work vs heat.
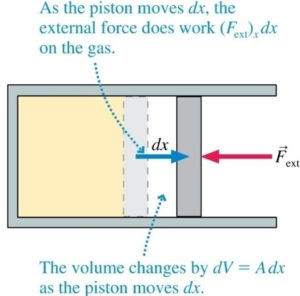
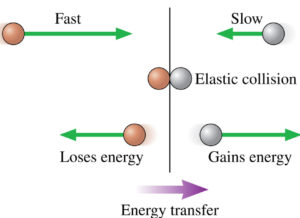
A: Work (W) and heat (Q) are the 2 ways to change the thermal energy of a system. They are different.
For Work, macro force/pressure and displacement/volume change are required, see figure.
Macroscopically, heat is an energy flow from one object to another which has no force/displacement/work involved.
Essentially, heat is a kinetic energy transfer due to Micro work done by one object on another in collisions, see figure.
For example, in an isochoric ideal gas process (volume is constant), work done is zero while heat is absorbed or released by the gas. In an adiabatic process, heat transferred is zero while work done by the gas is positive or negative.
